Find out more about Philips products in clinical pathology
Developed with the aim to increase efficiency and collaboration in your clinical lab

As the first digital pathology solution in the U.S. to be marketed for primary diagnostic use, the Philips IntelliSite Pathology Solution1 can aid pathologists to view and interpret digital images1.

As a leader in digital pathology for over a decade, at Philips we have an extensive network of pathologists and laboratories who test, validate and inform our designs. Our high throughput and performance has been demonstrated worldwide for primary diagnosis2.

Digital pathology aims to reduce pressure on pathology services by streamlining the workflow and extending collaboration with the aim of increasing diagnostic confidence. Digital pathology also opens the future promise of computational pathology which aims to enhance diagnostic decision-making, improve efficiency, and gain new insights.
Demonstrated technology in time savings and throughput performance 2
96%

'I experience an increase in my productivity for routine diagnosis with PIPS'
100%

'Philips delivers a platform which is reliable as a microscope when doing diagnosis'
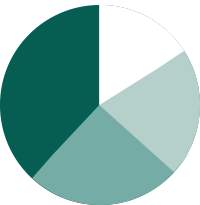

>10%

>20%

>15%

>25%
'What is your productivity gain due to Philips IntelliSite Pathology Solutions'

Enhanced patient care

Reduced costs due to efficiency and productivity gains
Improved workflow compared to analog

A proven solution that scales as you grow

The IMS case view simplifies access to histopathology cases

Understanding your needs
It’s about how we can help you and that we understand that your challenges will not be solved with products or technology alone. It’s about solving problems with integrated solutions and addressing healthcare industry-wide challenges.
Highlights of our selection of customer partnerships for primary diagnostics
LabPON
AZ Sint-Jan, Bruges
1. The Philips IntelliSite Pathology Solution has obtained market access clearance as IVD for primary diagnosis in approx. 50 countries, such as EEA (European Economic Area), USA, Canada, Japan, South Korea, and other countries in Asia, Middle East and South America. 2. Philips DCP research study, results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions (2018) 3. Collaboration Suite is not intended for diagnostic, monitoring or therapeutic purposes or in any other manner for regular medical practice.




