Find out more about Philips products in life sciences
Digital pathology in life sciences
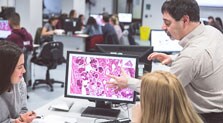
Biomarker and drug discovery are key in the development of new anticancer therapies. An increased urgency for clinical development has emerged, driven by growing cancer projections and low success rates in the lengthy and costly drug development pipeline. With a complete end-to-end solution in digital pathology offering high flexibility in image analysis and multi-site research, Philips aims to accelerate drug development throughout all phases of the pharmaceutic pipeline.
Supporting all stages of drug development
Our product portfolio supports biomarker and target discovery, tumor detection, effective tissue data management and analysis, multisite collaboration and shared workflows. By permitting data integration of dispersed pharma pathology labs around the world, we provide a solid basis for efficient and precise tissue and biomarker evaluation, as well as clinical trials in cancer research.

Drug & biomarker discovery
Pre clinical
I – III
Review & approval
Post
market
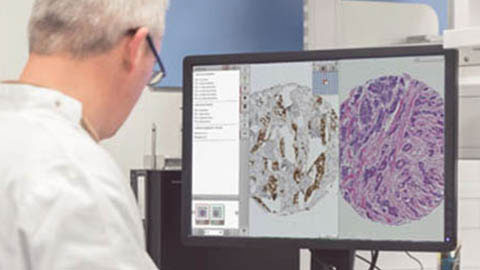
Biomarker & drug discovery
Tissue-based human and animal research to identify biomarkers and drug targets.
Philips Xplore1 allows you to integrate and analyze multisource research data in an open and highly flexible data platform. Design studies around any key variable and score tissue microarrays and whole slide images.
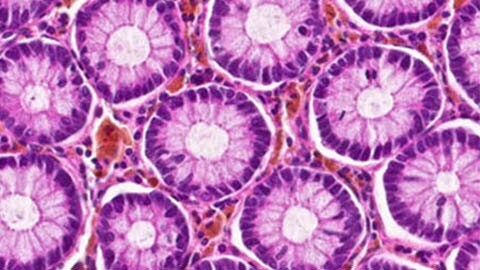
Preclinical studies
Tests of drug-target interactions, toxicity and dose finding in animal and human tissue.
Measure abnormalities in tissue after exposure to new compounds.

Clinical trials & post market
Evaluate the effectivity, safety, and dose ranging in selected subjects and patient groups.
With shared workflows, collaborated image evaluation, and connected teams, we promote the harmonization of procedures atnd data collection that is crucial for global multicenter clinical studies, especially for safety monitoring boards or adjudication committees.

Training & quality assessments
Throughout the entire drug-to-market process, Tutor3 presents an education and training platform for harmonization, training, as well as External Quality Assessment during biomarker and assay development.
Tutor enables Life Sciences to fast track training and speed up review processes. We connect teams by accommodating digital workflows and remote consultation with our Collaboration Suite4.
Advancing precision medicine
With our end-to-end solution in digital pathology, philips aims to support health care providers to practice precision medicine and target the right treatments to the right patients at the right time.
Tissue pathology is at the heart of various aspects of drug development from biomarker and drug discovery to post-market clinical studies. Biomarker analytics and tissue characterization and are the keystones of anticancer drug discovery and diagnostics.