The number of image-guided interventional oncology procedures is growing. The procedures are minimally invasive and are often a good option for cancer patients that are not eligible for surgery, chemotherapy or radiation therapy. Around 80,000 interventional oncology procedures were performed in 2013 in the US alone, a figure expected to double by 2023.¹ But to fulfill the promise of interventional oncology, clinicians must have an excellent view of the treatment targets for informed decision making and the best chances of reducing the impact on healthy tissue.
Philips OncoSuite, discussed in an earlier edition of HotSpot, is a comprehensive interventional oncology portfolio to help clinicians segment tumors and to plan and guide interventional procedures. In September 2016, Philips unveiled the next generation OncoSuite platform that will enable physicians to provide targeted treatment of tumor and its feeder vessels without affecting surrounding tissue or organs.
OncoSuite - an integrated solution for tumor embolization and ablation
OncoSuite consists of Philips’ XperCT Dual, EmboGuide and XperGuide tools for treatment planning and guidance. It is the only platform in the industry that supports both tumor embolization and ablation and that enables physicians to target multiple tumor lesions simultaneously. Offered with Philips interventional X-ray systems, OncoSuite can be used for treating tumors in various organs such as the liver, bone, kidney and lung. The latest edition of OncoSuite was launched at the 2016 Cardiovascular and Interventional Radiological Society of Europe annual meeting (CIRSE 2016), where it gathered much interest.
XperCT Open Trajectory - for better centering of the liver
Liver cancer is the second leading cause of cancer deaths in the world, and one of the most challenging to treat.² However, traditional geometric movement of the C-arm centers the field of view on the spine, which sometimes fails to capture the entire liver. The new Open Trajectory feature in OncoSuite 2.0 opens the rotation arc to the left side of the patient.
Instead of the traditional closed arc, which starts and stops at -120°/+120° degrees of rotation, Open Trajectory has a new start and stop position of +55° and -185°. This allows additional space to move the patient table to the side, so clinicians can center the field of view on off-centered organs of interest such as the liver. This new feature of XperCT Dual allows liver-centered imaging in a single sweep, with significantly improved intra-procedural depiction of peripheral hepatic tumors.
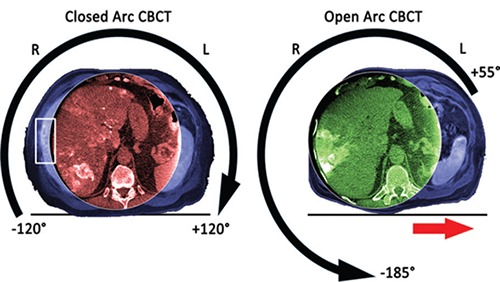
The difference in liver coverage between a standard closed trajectory (left) and the new Open Trajectory (right). In the closed trajectory image, part of the liver falls outside the imaging view while in the open trajectory image, the complete liver is visible, including the periphery.
XperCT for LUMI - world’s first optimized imaging for radiopaque beads
After embolization with radiopaque beads, clinicians can have lingering concerns about whether the tumor has been embolized completely and how much healthy tissue was affected. In OncoSuite 2.0, Philips is proud to offer a new feature that helps reduce these worries: the world’s first dedicated imaging protocol for radiopaque embolization beads. BTG, Biocompatibles UK Ltd., has introduced radiopaque beads for endovascular treatment procedures of the liver; LC Bead LUMIᵀᴹ in the US and DC Bead LUMIᵀᴹ in Europe respectively.
The supporting optimized 2D and 3D visualization protocols were developed by Philips as part of a joint development agreement with BTG. These protocols allow clinicians to visualize the accumulation of embolization beads in the tumor. This offers the opportunity for real-time adjustment during the procedure and an immediate visual post-procedural control that helps clinicians deliver treatment with more confidence.
With BTG’s radiopaque beads and Philips OncoSuite 2.0, clinicians can already see in the immediate post-procedural cone-beam CT scan whether the entire tumor has been embolized and where the embolization beads have ended up in healthy tissue. This may potentially help physicians to explore follow-up treatment options immediately, rather than to wait for the traditional 4 or 6-week post-treatment imaging.
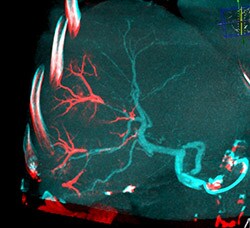
Dual View fusion of final CBCT with beads (red) overlaid on arterial non-selective CBCT (blue) clearly identifies the vascular regions targeted by the radiopaque beads (LC Bead LUMITM).
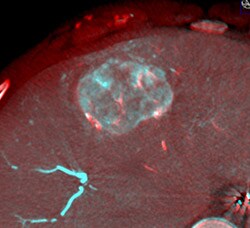
DualView image of fused pre-embolization XperCT with intra-arterial contrast and post-embolization XperCT. Blue color indicates patient vessels prior to embolization, while red overlay documents the presence of LC Bead LUMITM. Yellow arrow points to tumor vessel which has failed to accumulate embolization microbeads.
The next generation of interventional oncology tools, for even greater insight and confidence
The new features of OncoSuite, Open Trajectory and the optimized protocol for radiopaque beads, are designed to support the proven 3D guidance provided by EmboGuide and XperGuide tools. This next generation OncoSuite is better equipped than ever to help clinicians gain greater insights and confidence in interventional oncology treatment. With the combination of these new innovative features and our live image guidance, we aim to remove the barriers to safer, effective and reproducible minimally invasive treatments, delivering clinical value where it’s needed most – at the point of patient care.
References
1) Medtech 360 Report: Interventional Oncology Devices | US | 2015 | Market Analysis, 2015 Decision Resources Group.
2) World Health Organization (WHO). Cancer factsheet,
https://www.who.int/mediacentre/factsheets/fs297/en/.
*XperCT R3.3 / the new Open Trajectory and XperCT for LUMI options are not cleared yet in some countries
For more information
Read more about the Philips Interventional Oncology solutions here:
Written by:

Thiru Kanagasabapathi, PhD
Sr. Product Manager, iXR Clinical Solutions
